Researchers develop membrane-free electrochemical system for high-efficient and high-purity phosphate recovery
Phosphorus (P) is often regarded as the primary stimulant for eutrophication, while its vital role in industrial and environmental development is also well acknowledged. Given its future scarcity, P recycling from waste streams is suggested and practiced.
Assistant Professor Yang Lei’s team from the School of Environmental Science and Engineering at the Southern University of Science and Technology (SUSTech) has recently proposed a novel concept for the second generation of the CaCO3-packed electrochemical precipitation system: a basket-shaped anode electrochemical system for enhanced phosphorus recovery and product quality.
Their research, entitled “Basket anode filled with CaCO3 particles: A membrane-free electrochemical system for boosting phosphate recovery and product purity,” has been published in Water Research, a top journal covering all aspects of the science and technology of the anthropogenic water cycle, water quality, and its management worldwide.
In this research, Prof. Lei’s team constructed a basket-anode electrochemical system and validated it systematically in terms of phosphorus recovery efficiency, long-term stability, impacts of operational parameters, and the techno-economic feasibility of treating real urine.
Rule of the CaCO3-filled basket anode
Under a specified constant current, the reaction between anode-generated H+ and the packed CaCO3 particles conserves OH– and brings Ca2+ into the bulk solution, as confirmed by the increased pH and Ca2+ concentration (Figure 1B). The introduction of this novel basket anode allowed the CaCO3-H+ reaction to occur within the limited anodic area and increased the pH and Ca2+ concentration in the cathode region, which is ideal for inducing Ca-phosphate formation and precipitation (Figure 1A).
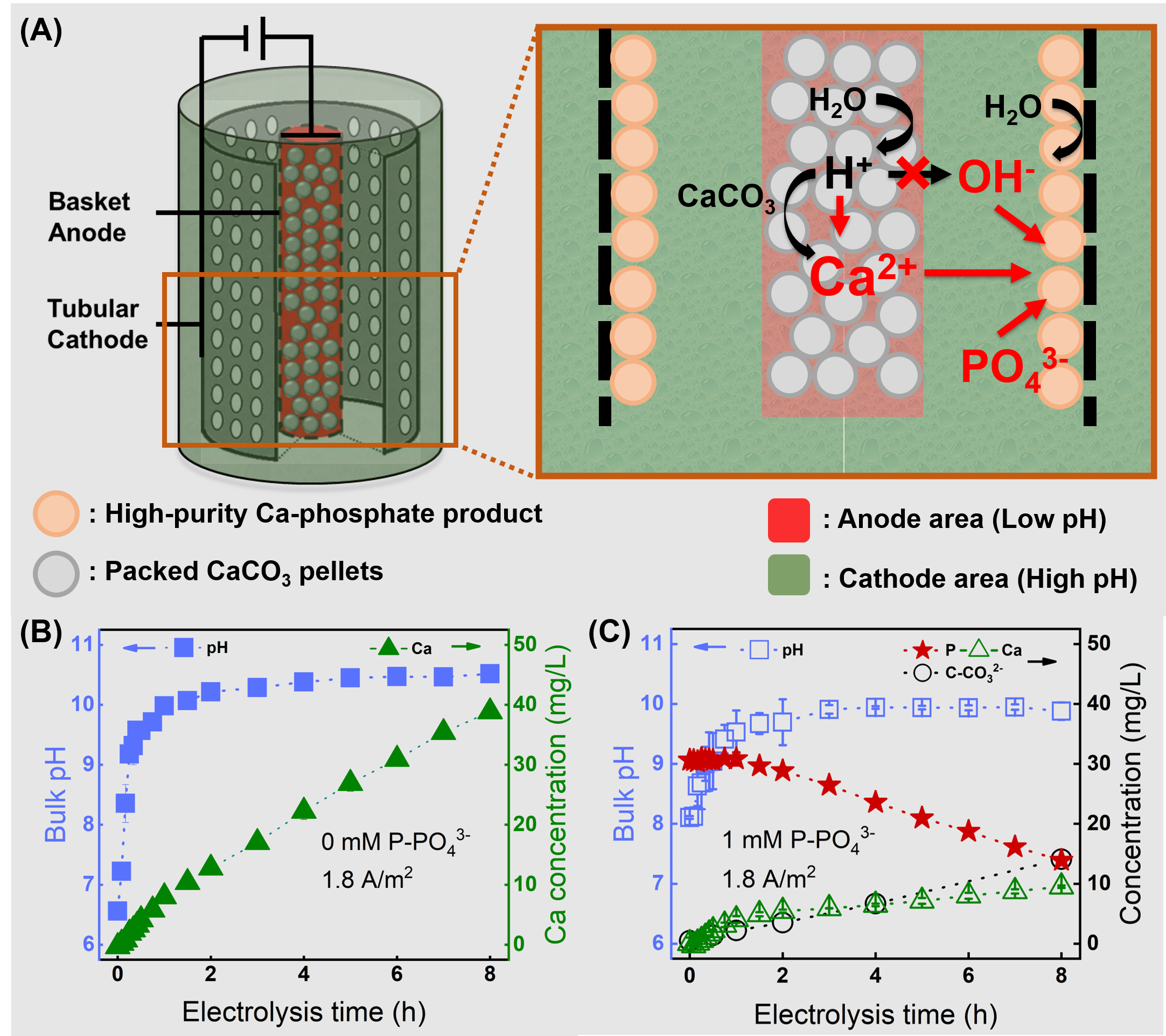
Figure 1. (A) Schematic diagram of separating the low-pH anodic and high-pH cathodic chamber in the membrane-less electrochemical system coupled with the basket anode. (B) The evolution of pH and Ca in the bulk solution over electrolysis. (C) Change of P, Ca, and inorganic carbon over the electrolysis.
Enhanced phosphorus recovery in this basket-anode electrochemical system
As confirmed above, the packed CaCO3 particles in the basket-shaped anode consumed the anodic produced H+, providing Ca2+ and enhancing the high pH environment beyond the cathode surface. Accordingly, when phosphorus is present in the feed solution, the system achieved a noticeable P removal of 55% (Figure 1C). Visible white precipitates were found on the cathode surface and were confirmed to be crystalline Ca-phosphate by SEM-EDS and XRD analysis.
Boosted product quality and stabilized performance over long-term operation
The novel structure of the basket anode, by its derived acidic anode region and alkaline cathode region (Figure 1A), completely avoids the precipitation of Ca-phosphate on the packed CaCO3. The researchers found that almost all the P was recovered as high-purity, highly crystalline Ca-phosphate on the cathode over the 70-day continuous operation (Figure 2I and 2J). The precipitation of P at the basket anode, packing material, and bulk solution was precluded by solid characterization and elemental analysis (Figure 2). This boosting effect on product purity is even more remarkable when they compare its P content (13.5 wt%) with that (0.1 wt%) in the first generation of the CaCO3-packed electrochemical precipitation column.
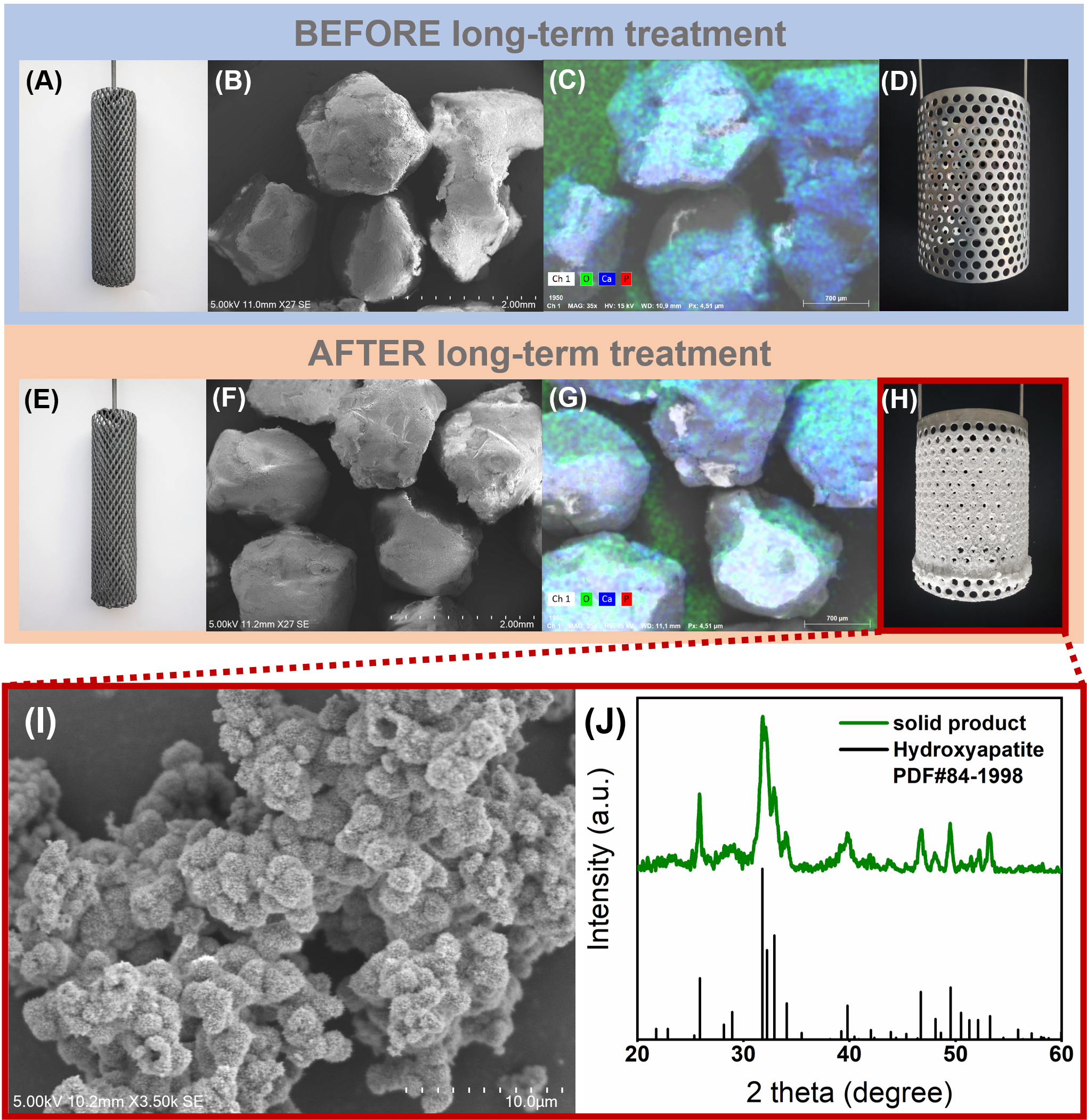
Figure 2. Photography of the basket anode (A) before and (E) after 70 days of electrolysis. SEM images of the packed pellets (B) before and (F) after 70 days of operation. Element mapping of the packed pellets (C) before and (G) after 70 days of operation. Photography of the stainless-steel cathode (D) before and (H) after 70 days of use. (I) SEM image and (J) XRD pattern of the solid products collected from the cathode side facing the basket anode after 70 days of long-term operation.
Influences of operating conditions on system’s performance
The applied current density, pellets size, and influent P concentration were all demonstrated to be critical for P removal performance, product purity, and power consumption. Enhancing the reaction between packing materials and anode-derived H+, such as increasing applied current or packing materials’ specific surface area, were all demonstrated to be promising ways to optimize the system performance.
Performance toward real wastewater and economic analysis
The researchers further explored the effectiveness of this system in treating actual P-rich wastewater (stored urine). 87% of P was recovered from stored urine as P-rich products ready to be collected from the cathode, at an energy consumption of 30 kWh/kg P comparable with other advanced electrochemical techniques (Figure 3). The economic analysis indicates that a gross profit of €0.16/m3 urine treated or €3.23/kg P recovered can be obtained.
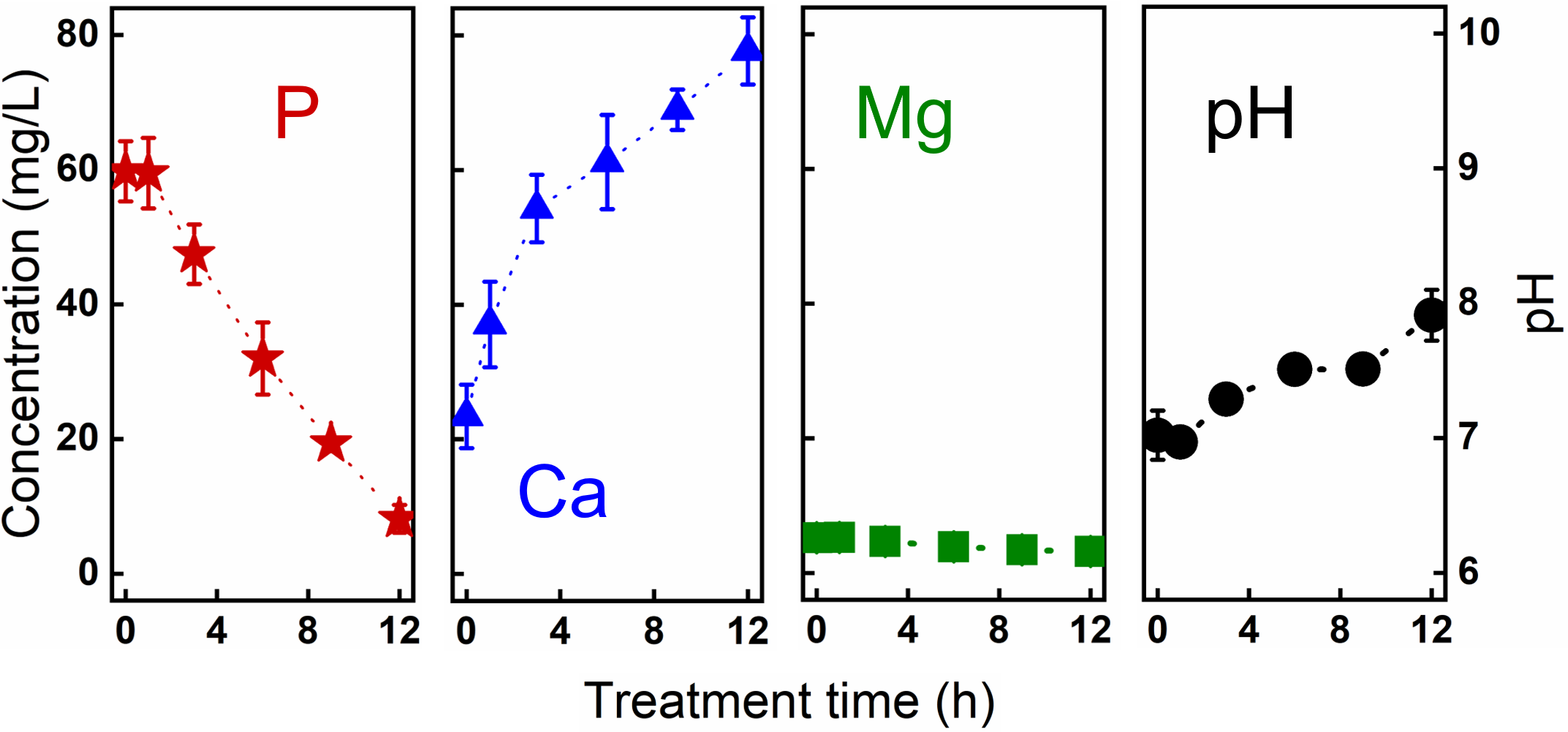
Figure 3. The P recovery performance and evolution of Ca, Mg, and pH in the basket anode coupled electrochemical system treating real stored urine.
Prof. Lei’s team results suggest that the CaCO3-packed basket anode proposed in this research plays a membrane-like role in the electrochemical system, dividing the system into a low-pH anode region and a high-pH cathode region, which greatly enhances the efficiency and product quality of wastewater phosphorus recovery. This research advances the development of electrochemical phosphate precipitation technology. Last but not least, the membrane-like function of the basket anode is expected to provide ideas for other water treatment technologies.
Zhengshuo Zhan, a Ph.D. student at SUSTech, is the first author of this paper. Asst. Prof. Yang Lei at SUSTech is the corresponding author. Prof. Michel Saakes from the European centre of excellence for sustainable water technology (Wetsus), along with Assoc. Prof. Renata D. van der Weijden and Prof. Cees J.N. Buisman from Wageningen University & Research also contributed to this study.
This research was supported by the Stable Support Plan Program of Shenzhen Natural Science Foundation and the SUSTech Start-Up Fund.
Paper link: https://doi.org/10.1016/j.watres.2023.119604
