南科大环境学院雷洋团队:电化学生物壳高值化构建固废处置、污水治理与资源回收一体化新策略
南科大环境学院雷洋团队:电化学生物壳高值化构建固废处置、污水治理与资源回收一体化新策略
近日,南方科技大学环境科学与工程学院助理教授雷洋团队在Environmental Science & Technology发表了题为“Electrochemical Upcycling of Shell Waste for Sustainable Nutrient Recovery from Wastewater”的研究论文。该研究提出一种创新的电化学策略,通过高值化废弃生物壳,实现污水中的氮磷资源化。此外,研究团队设计并搭建了适用于家庭场景的中试装置,用于废弃生物壳高值化与尿液磷肥生产,显著降低了处理成本与碳排放,为可持续固废处置与污水治理提供了新思路。
全球人口增长推动氮磷肥料需求持续上升,同时也加剧了食品加工副产物(如各类生物壳)排放与水体氮磷污染的双重挑战。研究团队提出了一种电化学策略,通过高值化废弃生物壳实现污水中的氮磷回收,以综合应对上述挑战。
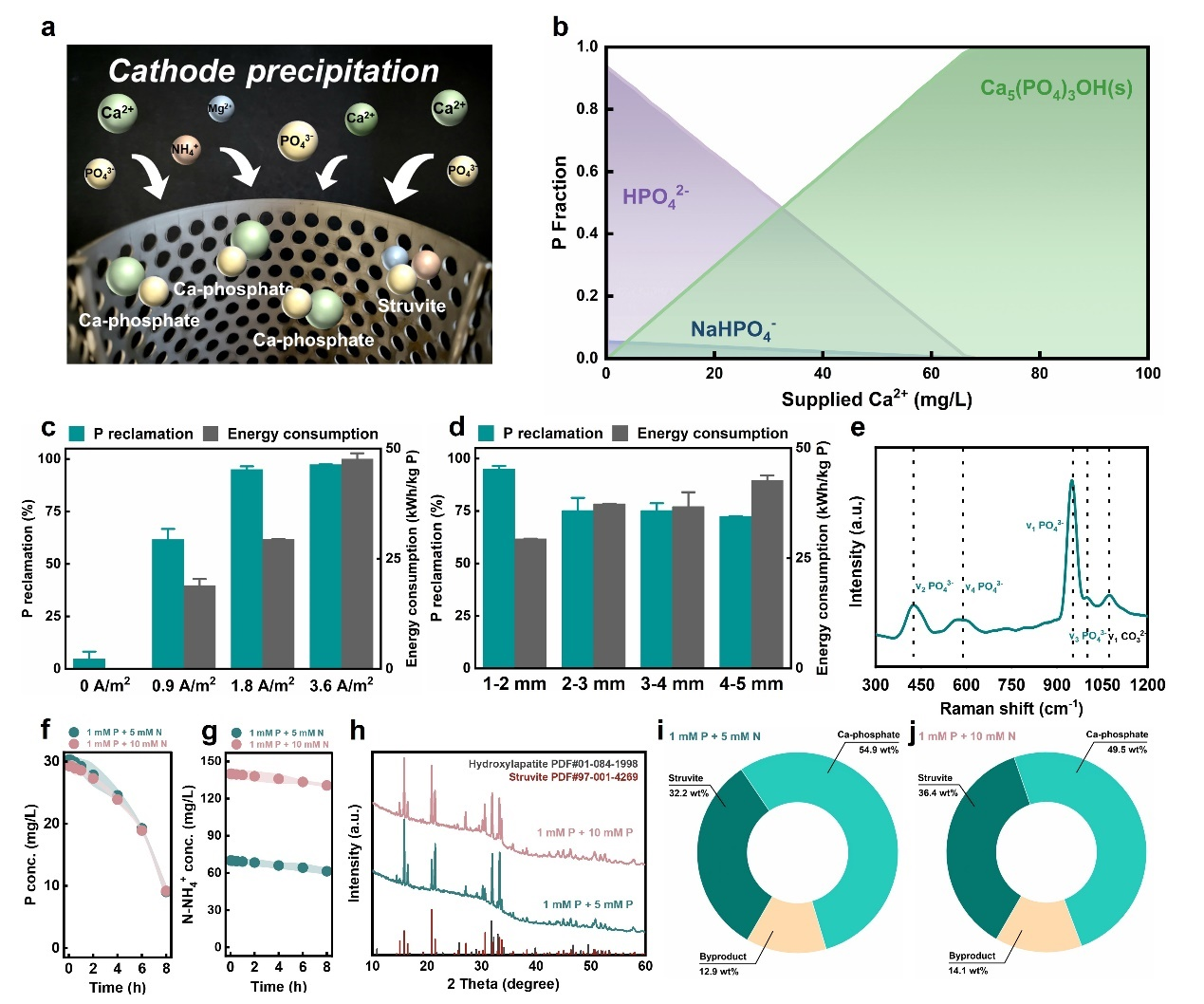
图 1 (a)阴极诱导氮磷沉淀反应机理图;(b)热力学模拟;(c-d)电流密度(c)及壳类破碎程度(d)于磷回收率及比能耗的影响;(e)磷酸钙产物的拉曼光谱;(f-g)蜗牛壳高值化过程中磷(f)及氮(g)浓度变化;(h)鸟粪石产物XRD图谱(i-j)不同污水N/P比下回收产物成分分析。
该策略的核心在于阳极的水氧化反应生成H+,形成局部酸性环境,驱动废弃生物壳的分解。以牡蛎壳为例,其分解后释放的钙离子(Ca2+),耦合阴极产生的局部碱性环境,促使污水中的磷酸根(PO43-)以磷酸钙的形式结晶回收(图1a-e)。在此框架下,特定含镁生物壳(如蜗牛壳)可通过释放自身含有的镁离子(Mg2+),进一步实现了磷酸根(PO43-)与铵根(NH4+)以鸟粪石(MgNH4PO4)的形式共回收(图1f-j)。在资源回收的基础上,团队发现并证实,该高值化过程通过构建高浓度Ca2+/Mg2+及高碱度环境,展现出额外的二氧化碳捕获能力。
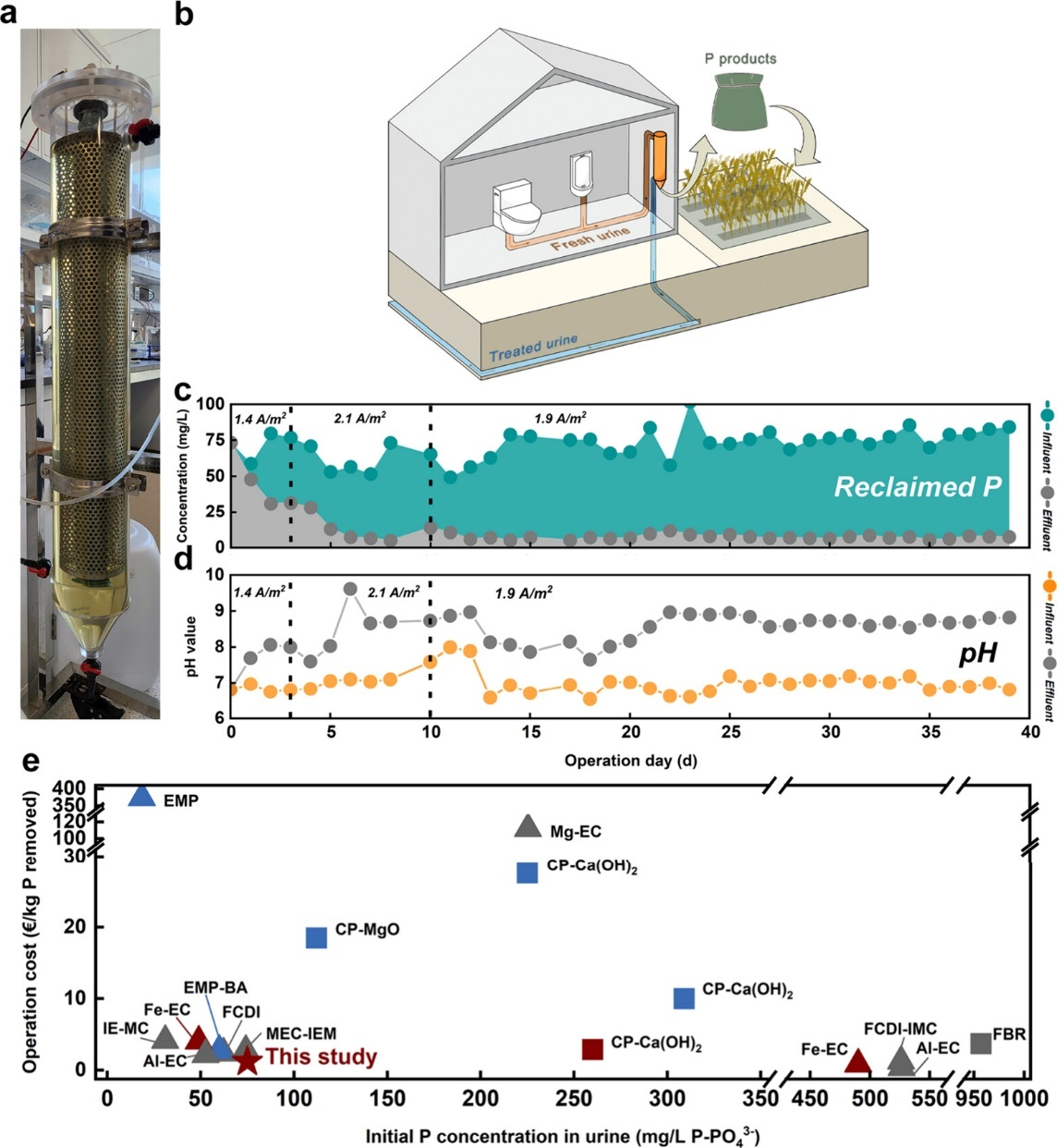
图2 (a) 家庭式中试装置;(b) “生物壳回用-尿液处理-肥料回收”的家庭式模式示意图;(c-d)30天运行期间的磷回收性能(c)及pH变化(d);(e)现存尿液磷回收工作的归一化运行成本对比。
为验证该策略的实际应用潜力,研究团队设计并构建了中试装置,用于家庭场景下的生物壳高值化及尿液氮磷资源化(图2a-b)。该装置表现出稳定且优异的磷回收性能(1.0 kW h/m3,磷回收率>85.7%)(图2c-e),且回收产物具有高磷含量(13.1 wt%)及高生物可利用性(93.3%),符合肥料回用标准。这一“生物壳回用-尿液处理-肥料回收”的家庭式创新模式,已被证实能够带来显著的碳减排及经济效益。
南方科技大学为论文第一和通讯单位,环境科学与工程学院博士研究生詹铮铄为第一作者,雷洋为通讯作者,课题组成员罗家钰与李炜权为研究做出了重要贡献。合作者包括南方科技大学讲席教授刘崇炫,欧洲水研究中心(Wetsus)研究员Michel Saakes,瓦赫宁根大学博士刘霁瑶、Renata D. van der Weijden教授和Cees Buisman院士。该研究得到了广东省基础与应用基础研究基金、深圳市科技计划项目以及高水平专项资金的联合资助。
复制下方论文链接至浏览器访问或点击文末“阅读原文”,即可查看论文:
https://pubs.acs.org/doi/full/10.1021/acs.est.5c09352






