Researchers reveal dynamic behavior of cadmium and carbon during transformation of ferrihydrite
Cadmium (Cd) is considered an environmental pollutant, which often accumulates in rice fields. How to remediate and manage soil containing cadmium is a hot issue in China. Iron (Fe) oxides and fulvic acid (FA) are the key components affecting the fate of cadmium in soil.
The presence of FA influences Fe mineral transformation, and FA may complicate phase transformation and the dynamic behavior of Cd. However, there is still a lack of quantitative understanding of how varying Fe minerals and FA affect Cd immobilization during the ferrihydrite transformation induced by various Fe(II) concentrations, however, is still lack of quantitative understanding.
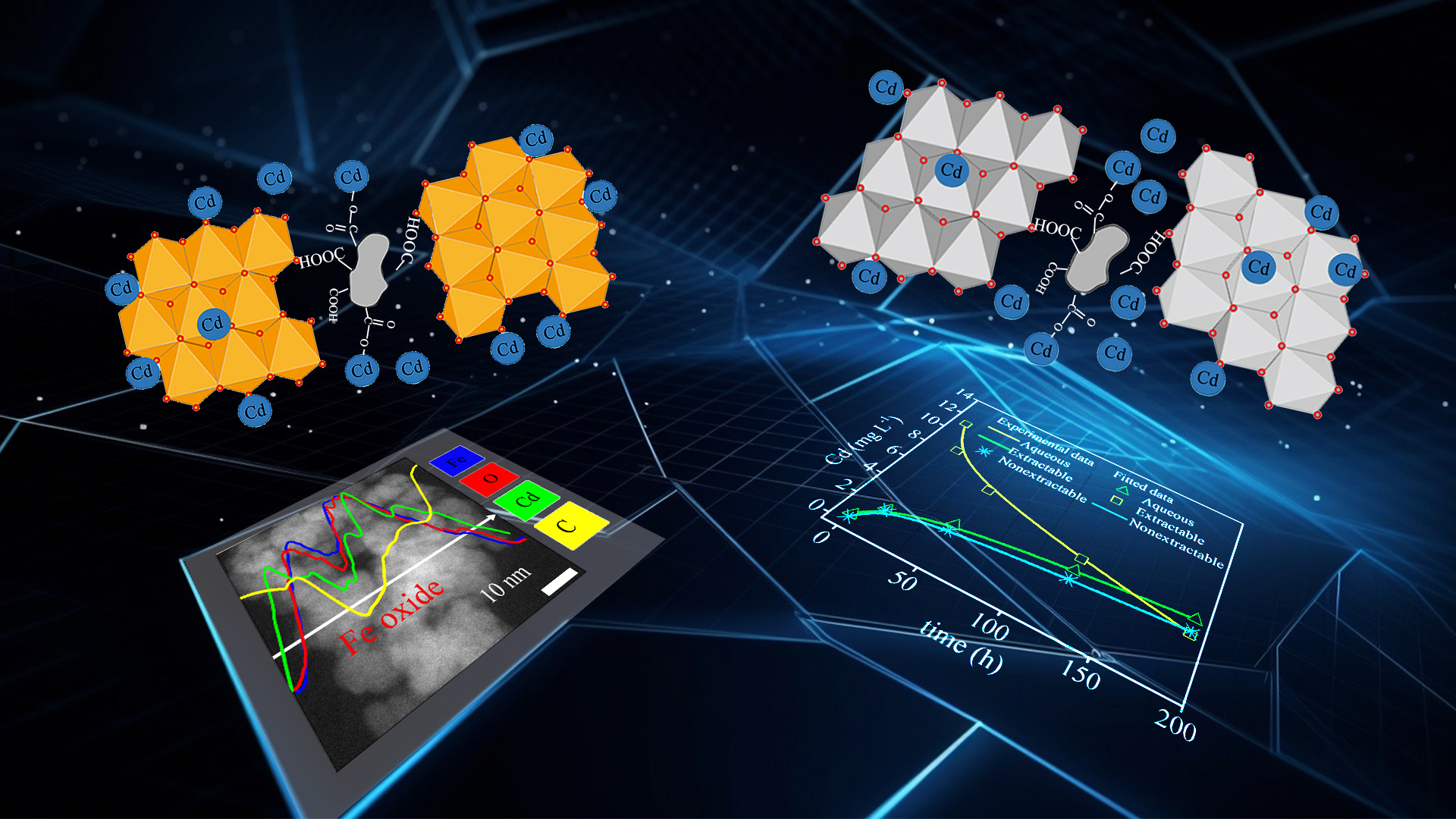
Xinyue Shen, an undergraduate student of Chair Professor Chongxuan Liu’s research team from the School of Environmental Science and Engineering (ESE) at the Southern University of Science and Technology (SUSTech), has recently published a paper that built a model for Cd species quantification during phase transformation based on mechanistic insights obtained from batch experiments.
The paper, entitled “Mechanistic and modeling insights into the immobilization of Cd and organic carbon during abiotic transformation of ferrihydrite induced by Fe (II)”, has been published in the Journal of Hazardous Materials, an internationally renowned journal in the field of environmental science and ecology.
Ferrihydrite, a poorly crystalline Fe mineral in soil, can occur phase transformation and transform into other secondary Fe minerals. However, in soils, ferrihydrite usually exists in the form of complexes with organic matter (OM), which complicates the reaction of ferrihydrite and heavy metals. Cadmium, a typical heavy metal in anoxic soil environments, can be fixed by complexes of ferrihydrite and OM through adsorption and co-precipitation. The coprecipitates of ferrihydrite, Cd, and OM (Fh-Cd-OM) are thermodynamically unstable and undergo a phase transformation under the influence of Fe(II).
In this study, the dynamic behaviors of Cd during Fe(II) induced ferrihydrite transformation were investigated through batch experiments, a series of advanced characterizations, and a quantitative model. The XRD and XAS results showed that the activity of Fe(II) redistributed ferrihydrite-associated Cd into more stable Fe oxides, most probably magnetite at high Fe(II) concentration and lepidocrocite at low Fe(II) concentration. The EDS and EELS analyses revealed that no incorporated FA was formed. In contrast, Cd may be immobilized by newly-formed Fe oxides through physical isolation, surface complexation, and incorporation.
These findings expand our understanding of the behavior of Cd at the interfaces of Fe oxide-OM and help develop a predictive model for Cd cycling by coupling other redox and chemical processes.
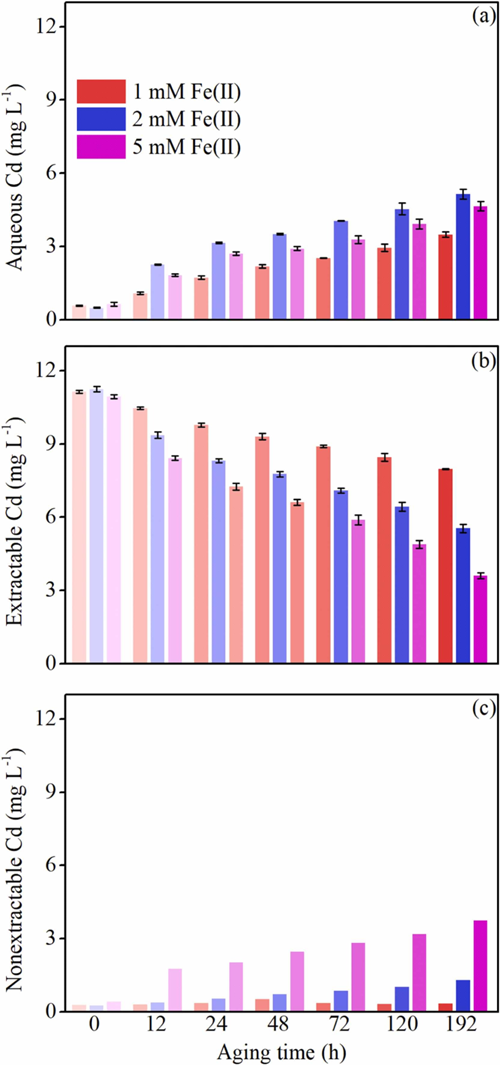
Figure 1. Time-dependent of (a) aqueous Cd, (b) extractable Cd, and (c) nonextractable Cd concentrations during Fe(II)-catalyzed ferrihydrite transformation quantified by batch extraction experiments.
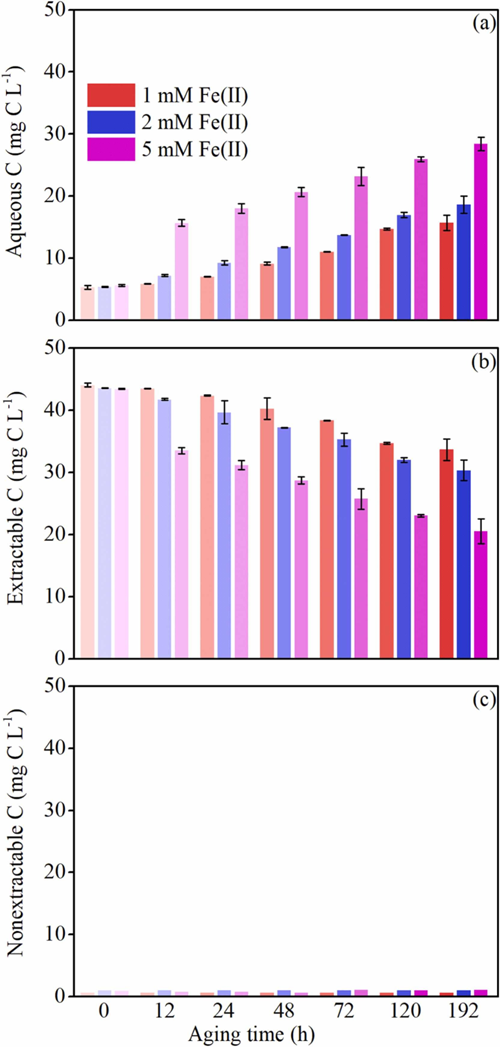
Figure 2. Time-dependent of (a) aqueous C, (b) extractable C, and (c) nonextractable C concentrations during Fe(II)-catalyzed ferrihydrite transformation quantified by batch extraction experiments.
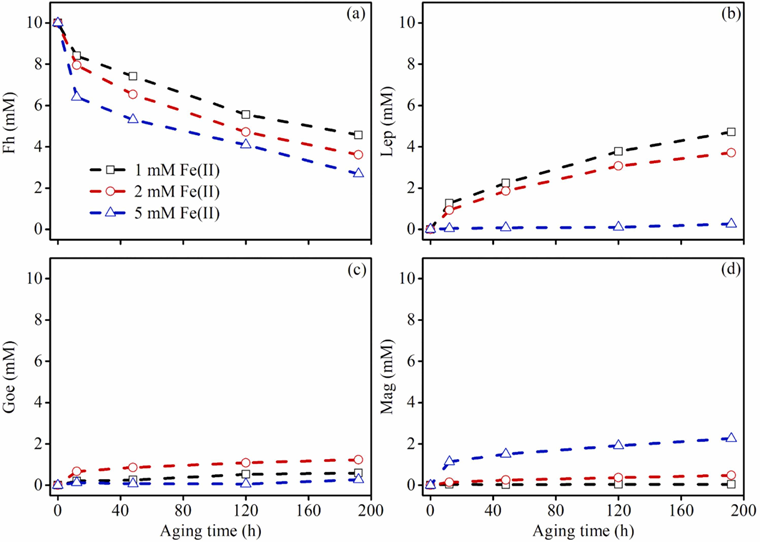
Figure 3. The concentrations of Fe oxide formed at various aging times determined by EXAFS LCF analysis for Fh-Cd-FA treatments. (a) ferrihydrite, (b) lepidocrocite, (c) goethite, and (d) magnetite.
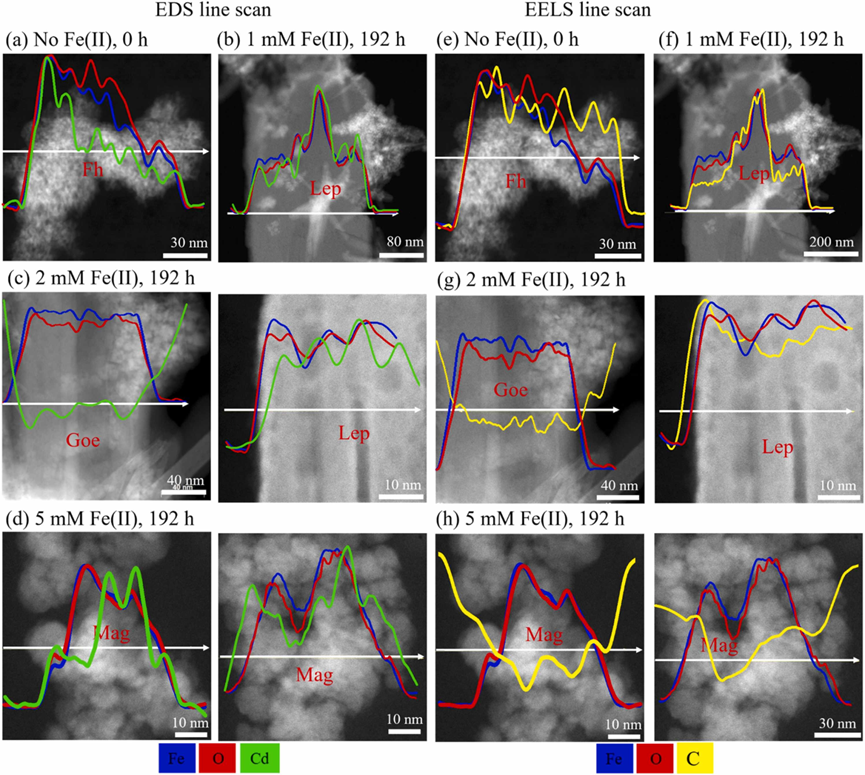
Figure 4. EDS and EELS line scans of Fe(blue), O(red), Cd(green), and C(yellow) in Fh-Cd-FA treatments.
Undergraduate student Xinyue Shen and master’s student Huiyan Zhu from the School of ESE at SUSTech are the co-first authors of this paper. Chair Professor Chongxuan Liu and postdoctoral student Shiwen Hu are the corresponding authors, and SUSTech is the first affiliation of the paper.
This work was supported by the Guangdong Basic and Applied Basic Research Foundation, China Postdoctoral Science Foundation, and the National Natural Science Foundation of China (NSFC).
Paper link: https://www.sciencedirect.com/science/article/pii/S0304389422010068
