Researchers develop new system for CO2 capture materials
With the intensification of human activities, CO2 emissions have surged, exacerbating the greenhouse effect and global warming. Carbon capture, utilization, and storage (CCUS) is considered the most effective and practical short-term solution for reducing CO2 emissions. According to the International Energy Agency, CCUS technology is expected to cumulatively capture 27 billion tons of CO2 by 2050. The development of novel, efficient CO2 capture materials is therefore of paramount importance.
In the quest for new materials, porous solid adsorbents have emerged at the forefront of next-generation carbon capture technology due to their non-corrosive nature and low regeneration energy consumption. Specifically, amine-functionalized adsorbents are suitable for various CO2 capture scenarios due to their high selectivity for CO2 and resistance to water vapor. However, these adsorbents still face challenges in practical applications, such as low adsorption efficiency and unstable cyclic performance. Thus, developing an efficient and stable CO2 capture material is crucial in addressing climate change challenges.
Professor Zuotai Zhang’s research team from the School of Environmental Science and Engineering at the Southern University of Science and Technology (SUSTech) has recently made a significant breakthrough in the field of CO2 capture. In their work, they introduced an innovative amine-support system that achieves efficient and stable CO2 capture, offering a new approach to the design of CO2 capture materials.
Their findings, entitled “Atom-level Interaction Design Between Amines and Support for Achieving Efficient and Stable CO2 Capture”, have been published in the prestigious journal Nature Communications.
The research team successfully synthesized a novel amine-support system based on atomic-level design. This system involves the precise impregnation of polyethyleneimine (PEI) molecules into the cage-like pores of MIL-101(Cr), forming strong coordination with the unsaturated chromium sites within, resulting in a stable composite material. This unique design not only achieves low regeneration energy (39.6 kJ/mol CO2) but also demonstrates excellent cyclic stability (only 0.18% decay per cycle under dry CO2 regeneration conditions), high CO2 adsorption capacity (4.0 mmol/g), and rapid adsorption kinetics (saturation achieved within 15 minutes at room temperature).
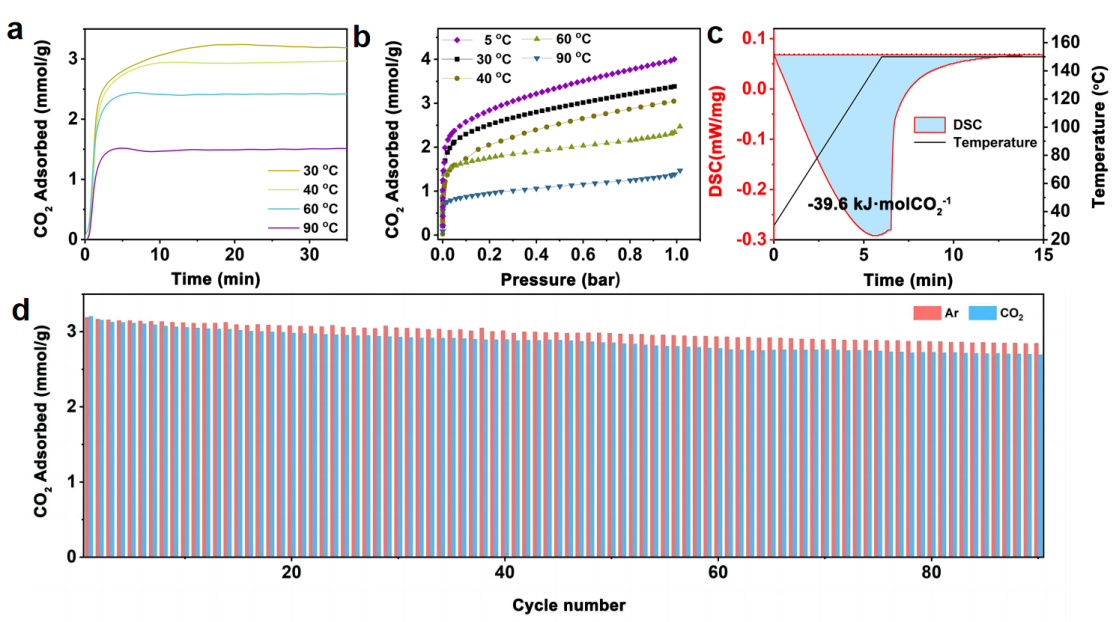
Figure 1. CO2 Capture performance of the novel amine-support system
Additionally, the study is the first to reveal the electronic-level interactions between amines and the support, preventing the dehydration reaction of carbamate products and significantly enhancing the material’s stability. The results indicate that this novel material has tremendous potential for CO2 capture, providing a feasible strategy for achieving low-cost, sustainable CO2 capture.
This work not only offers a new option for CO2 capture, but also provides essential references for future studies in environmental protection and energy conservation. The research team will continue to optimize and expand the carbon capture material system and explore its potential in practical industrial applications, contributing to global efforts to combat climate change.
Ph.D. candidate Xin Sun from Professor Zuotai Zhang’s group is the first author of the paper. Professors Zuotai Zhang and Xuehua Shen from SUSTech, and Hao Wang from Shenzhen Polytechnic University, are the co-corresponding authors. SUSTech is the first affiliated unit of the paper.
The study was supported by the National Natural Science Foundation of China (NSFC), Department of Science and Technology of Guangdong Province, and the Shenzhen Science and Technology Innovation Commission.
Paper link: https://www.nature.com/articles/s41467-024-48994-8
